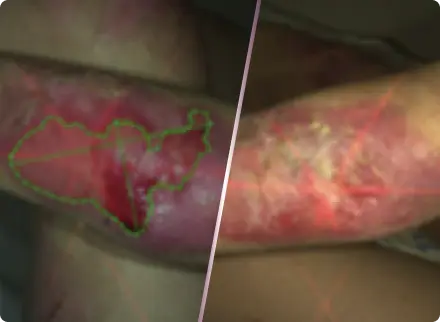
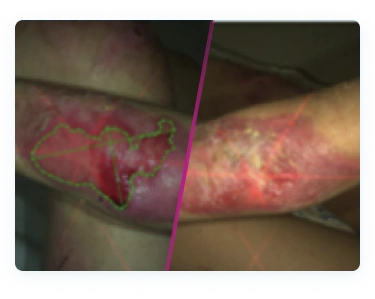
The most common side effect of FILSUVEZ is application site reactions, such as pain and itchy skin1
(n=109) n (%)
(n=114) n (%)
(N=223) N (%)
- The incidence of application site reactions with FILSUVEZ was comparable to placebo gel1
- Local hypersensitivity and skin reactions have been reported in patients treated with FILSUVEZ, including urticaria and dermatitis1
- Long-term treatment with FILSUVEZ was well tolerated, with no new safety signals identified at the end of the 2-year extension period2
- Patients using FILSUVEZ experienced a low incidence of wound infections, which were typically mild to moderate through the long-term analysis2