

FILSUVEZ demonstrated efficacy and safety in the landmark EASE trial1,2*
- Clinical trial design
- Primary end point
- Pediatric subgroup
- Open-label, follow-up study
- Before and after images
- Additional data
Clinical trial design
With 223 patients (195 with DEB and 26 with JEB), EASE was the largest phase 3 EB clinical trial to date1,2†
90-day, double-blind, phase 3 study3
All wounds, including target wounds, were treated with FILSUVEZ or placebo gel
FILSUVEZ + standard-of-care wound dressing‡ (109 participants)
Placebo gel + standard-of-care wound dressing‡ (114 participants)
Day 0
Day 45
Primary end point
Day 90
Day 0 of follow-up
2-year, open-label, follow-up study3
More than 90% of participants voluntarily continued into the extension, during which FILSUVEZ was applied to all wounds
FILSUVEZ + standard-of-care wound dressing‡ (205 participants)
Month 12
Year 2
End of
follow-up
follow-up
*The EASE trial was a global, phase 3, randomized, double-blind, placebo-controlled study.2,3
†Two patients with EB simplex were not included in study results.1
‡Standard-of-care dressings were nonadhesive dressings of physician or patient choice.2
Primary end point2
The proportion of patients with first complete closure of the target wound by Day 45
Key secondary (confirmatory) end point2
Time to first complete target wound closure (up to Day 90)
Key secondary (exploratory) end points2§¶
- Proportion with first complete target wound closure by Day 90
- Incidence of wound infection up to Day 90
- Maximum severity of wound infection between baseline and Day 90
- Change from baseline in total body wound burden (EBDASI) at Day 90
- Change from baseline in itching at Day 90
§Secondary end points were tested hierarchically. A nonsignificant result was achieved on the first key secondary end point; therefore, all subsequent end points were considered exploratory, not confirmatory.2
¶Additional secondary end points were also examined.2
EBDASI, Epidermolysis Bullosa Disease Activity and Scarring Index.
Complete closure criteria: A target wound selected in advance had to be 100% closed by Day 45 for treatment to be considered successful2
Clinical success2
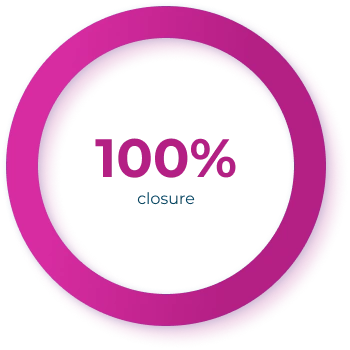
Complete wound closure was defined as skin re-epithelialization without drainage
Clinical failure2

Wounds that did not meet the definition of complete closure by Day 45 were classified as clinical failures regardless of visible progress
Primary end point
Double-blind phase
FILSUVEZ was proven to deliver complete wound closure# by Day 45 for more than 40% of patients1,2
More patients experienced 100% wound closure
with FILSUVEZ vs placebo gel by
with FILSUVEZ vs placebo gel by


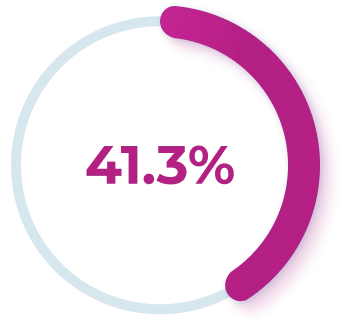

Patients using FILSUVEZ
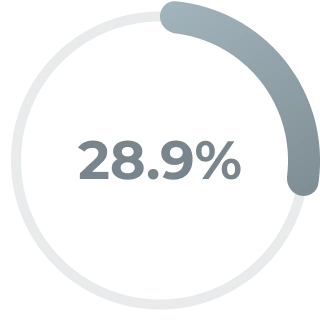

Patients using placebo gel
(RR, 1.44; 95% CI, 1.01-2.05; P=.013)
(RR, 1.44; 95% CI, 1.01-2.05; P=.013)
#Complete closure criteria: A target wound selected in advance had to be 100% closed by Day 45 for treatment to be considered successful. Complete wound closure was defined as skin re-epithelialization without drainage. Wounds that did not meet the definition of complete closure by Day 45 were classified as clinical failures, regardless of visible progress.2
CI, confidence interval; RR, relative risk.
CI, confidence interval; RR, relative risk.
Pediatric subgroup
Double-blind phase
In the pediatric subgroup, 70% greater target wound closure‖ probability with FILSUVEZ versus placebo gel4
More pediatric patients experienced
100% wound closure by Day 45
100% wound closure by Day 45
44.6%
Patients using FILSUVEZ

25.6%
Patients using placebo gel(RR, 1.70; 95% CI, 1.11-2.60)
.webp)
.webp)
‖Complete closure criteria: A target wound selected in advance had to be 100% closed by Day 45 for treatment to be considered successful. Complete wound closure was defined as skin re-epithelialization without drainage. Wounds that did not meet the definition of complete closure by Day 45 were classified as clinical failures, regardless of visible progress.2
CI, confidence interval; RR, relative risk.
Double-blind phase Open-label, follow-up study
FILSUVEZ provided reductions in total wound area** at Day 90 that were sustained over 2 years3††



Baseline
90 days
36% reduction in total wound area at Day 90 among patients using FILSUVEZ in the double-blind phase.2‡‡
Mean BSAP at Day 90 was 7.4% (-4.3% absolute change from baseline).2‡‡
2 years
50% reduction after 2 years of treatment among patients who continued using FILSUVEZ in the open-label, follow-up.3‡‡
Mean BSAP decreased from 12.1% at baseline to 6.1% at study completion.3‡‡
**Total wound area is defined as the percentage of body surface area affected by wounds.3
††Results are considered exploratory.2
‡‡Due to nonsignificant results on end points higher in the testing hierarchy, these results are observational in nature and should be interpreted with caution.2
BSAP, body surface area percentage. Before and after images
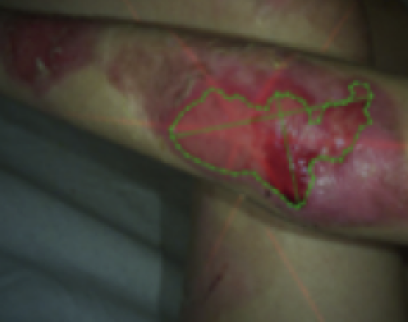
See the results after 3 months of treatment with FILSUVEZ3
Before

After
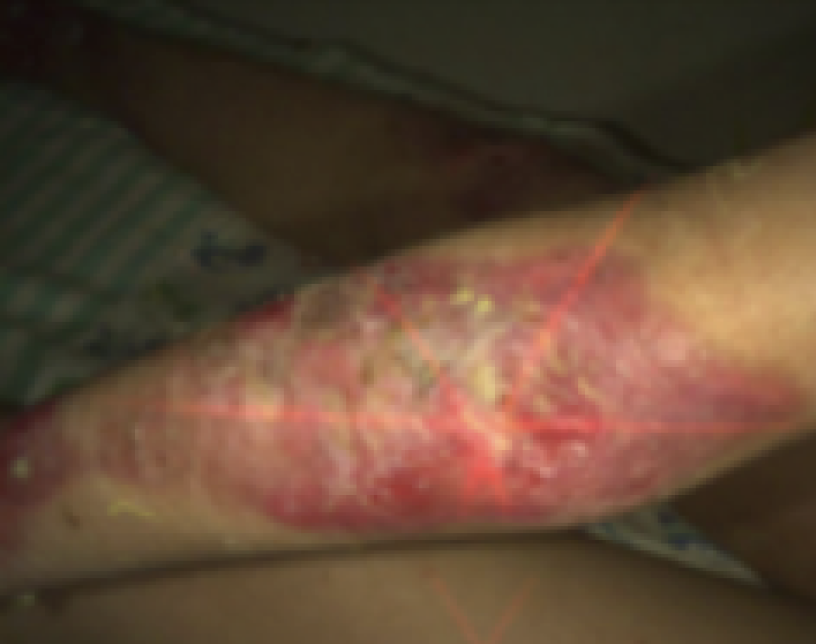

Before
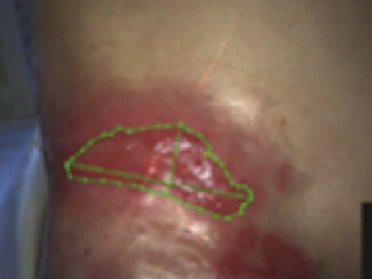

After
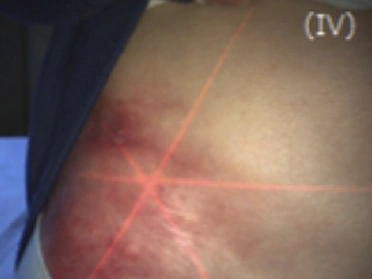

Before
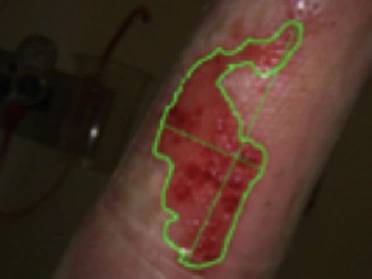

After
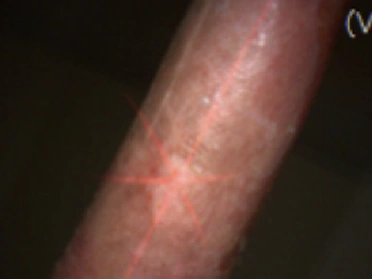

Before
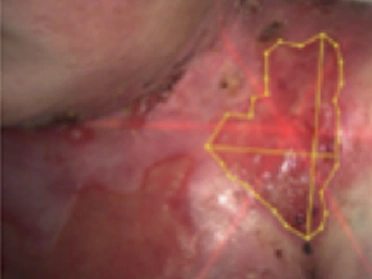

After
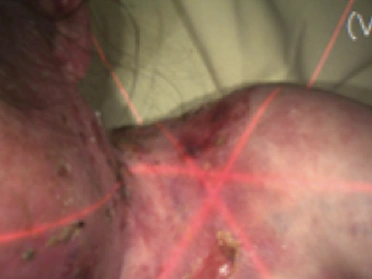

Before
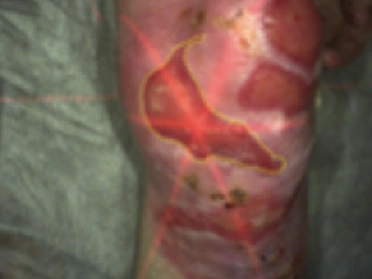

After
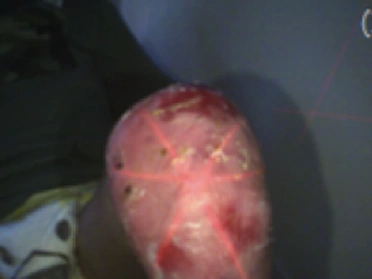

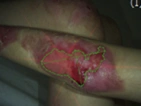

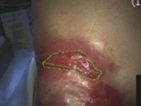

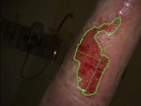

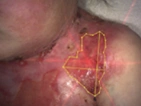

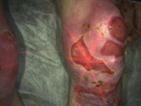

These images depict the wounds of real patients with recessive dystrophic EB from the EASE trial. Images used with permission.
Additional data
Post hoc analysis
In a post hoc analysis of a phase 3 trial, treatment with FILSUVEZ showed5:
In a subset of patients who required daily dressing changes:

change in the number of dressing changes required each week was:
FILSUVEZ (n=47) = -1.36
VS
Placebo gel (n=53) = -0.41
That amounts to nearly 3 fewer dressing changes every 2 weeks for FILSUVEZ vs 1 fewer for placebo gel.5§§
§§These data are derived from calculations based on a post hoc exploratory analysis. No formal statistical testing was planned and, therefore, no conclusions can be drawn.5
Explore the safety profile of FILSUVEZ
Safety


Henry, FILSUVEZ patient.


