DEB and JEB1
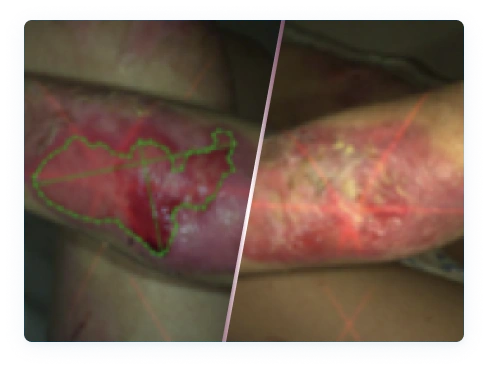
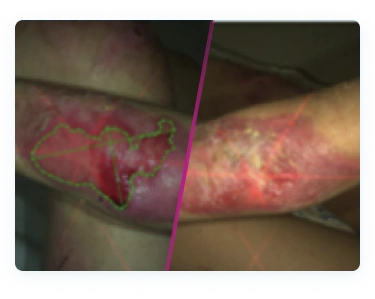






Local hypersensitivity and skin reactions have been reported in patients treated with FILSUVEZ, including urticaria and dermatitis. If signs or symptoms of hypersensitivity occur, discontinue use immediately and initiate appropriate therapy.
The most commonly reported adverse reaction in clinical trials was pruritus and pain at the wound application site (7.3%).
Please refer to Prescribing Information for administration instructions.
To report SUSPECTED ADVERSE REACTIONS, contact Chiesi USA Inc. at 1-888-661-9260 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see Full Prescribing Information.
Local hypersensitivity and skin reactions have been reported in patients treated with FILSUVEZ, including urticaria and dermatitis. If signs or symptoms of hypersensitivity occur, discontinue use immediately and initiate appropriate therapy.
The most commonly reported adverse reaction in clinical trials was pruritus and pain at the wound application site (7.3%).
Please refer to Prescribing Information for administration instructions.
To report SUSPECTED ADVERSE REACTIONS, contact Chiesi USA Inc. at 1-888-661-9260 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see Full Prescribing Information.
PP-RA-01344 V1.0