

Patients and caregivers can apply FILSUVEZ to all appropriate dystrophic or junctional epidermolysis bullosa wounds, at every dressing change, from the comfort of their own home1
- Applying FILSUVEZ
- Usage information
Applying FILSUVEZ
FILSUVEZ puts patients in control of their wound care with at-home application that integrates into existing EB wound care routines1
No clinic visits or healthcare providers are needed for routine application1
FILSUVEZ is applied in 3 steps1
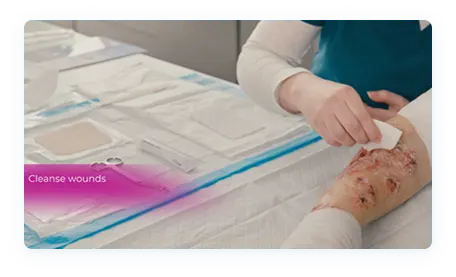
STEP 1
- Clean all wounds
- Using clean or gloved hands, open a new, single-use tube of FILSUVEZ
- Immediately after opening, apply 1 mm of FILSUVEZ evenly to each nonadhesive wound dressing, or directly to the wound. Once the tube has been opened and applied, discard the tube and any remaining gel
STEP 2
- Spread the 1 mm layer of FILSUVEZ across the entire wound surface to about the thickness of a credit card
- If applying to a dressing, spread FILSUVEZ across the entire dressing
.webp)
STEP 3
- Cover all wounds with sterile, nonadhesive wound dressings
- Reapply FILSUVEZ with each dressing change until all wounds are fully healed
- The tube of FILSUVEZ should only be used once
.webp)
Watch a wound care-certified nurse from the specialty pharmacy demonstrate proper FILSUVEZ application

Usage information
What to know about prescribing FILSUVEZ to patients with DEB or JEB
FILSUVEZ is supplied in easy-to-use, sterile, single-use tubes1

FILSUVEZ is available through PANTHERx, the specialty pharmacy

When applying FILSUVEZ directly to your wounds, a 1 mm layer (about the thickness of a credit card) should be applied evenly across the entire wound surface
.webp)
One tube covers the same area as a business envelope
Using FILSUVEZ1
.webp)
The tube of FILSUVEZ should only be used once. Once the tube has been opened, apply the gel to wounds immediately and then discard the tube and any remaining gel
.webp)
FILSUVEZ should be stored at room temperature between 68°F and 77°F (20°C to 25°C). Do not freeze
.webp)
Do not use FILSUVEZ in or around the eyes or mucous membranes (mouth, vagina, or anus). If accidental contact does occur, immediately wash the area with clean water and contact your doctor if you have any discomfort
Access tools and information to assist with prescribing FILSUVEZ
FILSUVEZ Support


